what is the electron configuration of aluminum|full electron configuration of platinum : Bacolod In order to write the Silicon electron configuration we first need to know the . Best Clash Royale Decks. Time. 1d. Total Games. 5,682 Size. 20 Sort. Rating Players. 1v1 Battle Type. Normal Battle Avg Elixir. 1 - 9 4-Card Cycle. 4 - 28 Refine . Include cards . Exclude cards . Reset. Apply card exclusion to both teams. Apply card filters . .
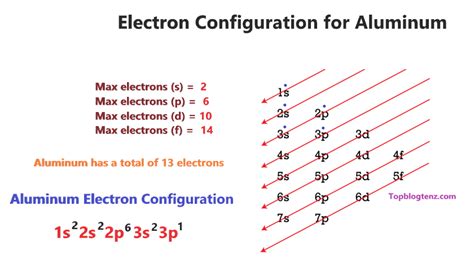
what is the electron configuration of aluminum,In order to write the Aluminium electron configuration we first need to know the number of electrons for the Al atom (there are 13 electrons). When we write the configuration we'll put all 13 electrons in orbitals around the nucleus of the Aluminium atom.
In order to write the Sulfur electron configuration we first need to know the .In order to write the Silicon electron configuration we first need to know the .
In order to write the Argon electron configuration we first need to know the .
When we write the configuration we'll put all 15 electrons in orbitals around the .How to Write the Electron Configuration for Carbon. Carbon is the sixth element with .
How to Write the Electron Configuration for Oxygen. Oxygen is the eighth element .When we write the configuration we'll put all 19 electrons in orbitals around the .In order to write the Mg electron configuration we first need to know the . Electron Configuration of Aluminum. To find the electron configuration of an atom, you first need to know the number of electrons that it has. Since aluminum's .
what is the electron configuration of aluminumThe Electron configuration of aluminum is 1s22s22p63s23p1. Aluminum is one of the elements that make up the periodic table, which is distinguished by its symbol Al, and its atomic number 13. A step-by-step description of how to write the electron configuration for Aluminum (Al). In order to write the Al electron configuration we first need to know the number of electrons for the.
What is the electron configuration of aluminum? Solution. Aluminum: Aluminum is a p-block element having an atomic number 13 and an atomic symbol Al. It belongs to .
Electron configuration The arrangements of electrons above the last (closed shell) noble gas. Melting point The temperature at which the solid–liquid phase change occurs. .The electron configuration of an atom is the representation of the arrangement of electrons distributed among the orbital shells and subshells. Commonly, the electron configuration is used to .Atomic properties. Electron configuration for aluminium. The history of Aluminium. Periodic table history. Identifiers. List of unique identifiers for Aluminium in various .Aluminum (Al) - Relative atomic mass (A) of aluminum is 27. Aluminum is a soft, silvery-white, ductile metal in the boron group. Learn more about properties, density and melting point of aluminium along with atomic . This tells you that the electron configuration of a neutral aluminium atom must account for a total of 13 electrons. The electron configuration of the neutral atom looks like this. Al: 1s22s22p63s23p1. Now, when aluminium forms 3 + cations, Al3+, it loses 3 electrons from its outermost energy shell. In this case, the 3 electrons will . The atomic number of aluminum is 13. A neutral atom of aluminum has 13 protons and 13 electrons. The ground state electron configuration for aluminum is 1s22s22p63s23p1. A shorthand way to write the electron configuration, called noble gas notation, is [Ne]323p1. An atom in its lowest energy state is said to be in its ground state.
The electron configuration for aluminum is 1s2, 2s2, 2p6, 3s2, 3p1. This is assuming the aluminum atom is a neutral atom in a grounded state.full electron configuration of platinum Electron configuration chart of all Elements is mentioned in the table below.The Shorthand electron configuration (or Noble gas configuration) as well as Full . Electron configuration of Aluminum (Al) [Ne] 3s 2 3p 1: 1s 2 2s 2 2p 6 3s 2 3p 1: 2, 8, 3: 14: Electron configuration of Silicon (Si) [Ne] 3s 2 3p 2: 1s 2 2s 2 2p 6 3s 2 3p 2:
what is the electron configuration of aluminum full electron configuration of platinum Electron configuration chart of all Elements is mentioned in the table below.The Shorthand electron configuration (or Noble gas configuration) as well as Full . Electron configuration of Aluminum (Al) [Ne] 3s 2 3p 1: 1s 2 2s 2 2p 6 3s 2 3p 1: 2, 8, 3: 14: Electron configuration of Silicon (Si) [Ne] 3s 2 3p 2: 1s 2 2s 2 2p 6 3s 2 3p 2: In this video we will write the electron configuration for Al 3+, the Aluminum ion. We’ll also look at why Aluminum forms a 3+ ion and how the electron confi.Electron configuration The arrangements of electrons above the last (closed shell) noble gas. Melting point . Aluminium is used in a huge variety of products including cans, foils, kitchen utensils, window frames, beer kegs and aeroplane parts. This is because of its particular properties. It has low density, is non-toxic, has a high thermal .They are helium, neon, argon, krypton, xenon, and radon. A noble gas configuration of an atom consists of the elemental symbol of the last noble gas prior to that atom, followed by the configuration of the remaining electrons. So for sodium, we make the substitution of [Ne] [ Ne] for the 1s22s22p6 1 s 2 2 s 2 2 p 6 part of the configuration.
The first two subshells of the third shell are filled in order—for example, the electron configuration of aluminum, with 13 electrons, is 1s 2 2s 2 2p 6 3s 2 3p 1. However, a curious thing happens after the 3p subshell is filled: the 4s subshell begins to fill before the 3d subshell does. In fact, the exact ordering of subshells becomes more .
The electron configuration and the orbital diagram are: Following hydrogen is the noble gas helium, which has an atomic number of 2. The helium atom contains two protons and two electrons. The first electron has the same four quantum numbers as the hydrogen atom electron ( n = 1, l = 0, ml = 0, ms = +1 2 m s = + 1 2 ). Add an electron to the anion electron configuration. For example, the ground state electronic configuration of chlorine is 1s²2s²2p⁶3s²3p⁵. For Cl −, it will be 1s²2s²2p⁶3s²3p⁶. Remove the outermost electrons in the cation, e.g. electron configuration for Mg 2+ will be 1s²2s²2p⁶.
The trick is to start at the next smallest noble gas and then complete the electron configuration as you normally would. Here is a video that explains further. Hope this helps! Answer link. [Ne] 3s^2 3p^1 The trick is to start at the next smallest noble gas and then complete the electron configuration as you normally would. Here is a video .What is the electron arrangement of aluminum? How many valence electrons does it have? Solution. Aluminum has 13 electrons so it will have the electron arrangement (2, 8, 3) which represents two electrons in the \(n=1\) energy level, eight electrons in the \(n=2\) level, and three electrons in the \(n=3\) level. Aluminum has three valence . The electron configuration shows the distribution of electrons into subshells. This list of electron configurations of elements contains all the elements in increasing order of atomic number.. To save . The first two subshells of the third shell are filled in order—for example, the electron configuration of aluminum, with 13 electrons, is 1s 2 2s 2 2p 6 3s 2 3p 1. However, a curious thing happens after the 3p subshell is filled: the 4s subshell begins to fill before the 3d subshell does. In fact, the exact ordering of subshells becomes more .
When their electron configurations are added to the table (Figure 6.29), we also see a periodic recurrence of similar electron configurations in the outer shells of these elements. Because they are in the outer shells of an atom, valence electrons play the most important role in chemical reactions. . Aluminum dication loses two electrons Al 2 .
The shorthand electron configuration for Aluminum is [Ne] 3s 2 3p 1. The electron configuration for the Aluminum ion (Al 3+ ) is 1s 2 2s 2 2p 6. The number of valence electrons available for the Aluminum atom is 3. Aluminum is situated in Group 13th or 3A and has an atomic number of 13. Given the periodic table: We see aluminum is atomic number 13. When we write our electron configurations, we utilize the valence orbitals relative to each period to construct it, and write a superscript for the maximum number of electrons in each orbital for each given period. So the full configuration is: Period 1 1s2 Period 2 2s22p6 Period 3 .

The electron configuration of an atom describes how its electrons are distributed among the available energy levels and orbitals. In the case of aluminium, the electron configuration can be represented as 1s^2 2s^2 2p^6 3s^2 3p^1. Let’s break this down: 1s^2: This represents the first energy level or shell, which can hold a maximum of .

The electron configuration of an atom describes how its electrons are distributed among the available energy levels and orbitals. In the case of aluminium, the electron configuration can be represented as 1s^2 2s^2 2p^6 3s^2 3p^1. Let’s break this down: 1s^2: This represents the first energy level or shell, which can hold a maximum of .
what is the electron configuration of aluminum|full electron configuration of platinum
PH0 · write an abbreviation configuration for aluminum
PH1 · full electron configuration of platinum
PH2 · electron configuration of all elements
PH3 · electron configuration guide
PH4 · electron configuration chart
PH5 · aluminum ground state electron configuration
PH6 · aluminum condensed electron configuration
PH7 · aluminum 3+ electron configuration
PH8 · Iba pa